Articles
More pH Probe Care and Usage Guidance
An interesting article on practical pH probe care was published in Water and Wastes Digest recently. It intrigued me enough to call one of the authors: Kareem Kay. He is the process reliability manager for Endress+Hauser who manufactures industrial pH equipment. Here are some key points from that conversation.
Temperature and temperature stress are probe killers. Temperature above 80C (175F) is a sure killer. In addition, shocking the probe with quick shifts from cool air to hot wort and back, is also a killer. Kareem confirmed that the recommendation to test only room-temperature wort is a good practice for long probe life.
Sometimes, the probe's bulb can get dirty. Don't touch the bulb with anything more than a soft bristle brush. He also pointed out that an acid soak or a detergent cleaning with the brush can restore a probe's performance. I will add that enzymatic detergents are probably best in brewery use since they are well-suited to working on the protein films that wort may produce.
Keeping the probe wet was a big point for him. If the bulb dries out, it does affect the performance. But he goes on to say that you might be able to rejuvenate a dried out probe by soaking it in potassium chloride solution. In fact, he further identified that his preferred probe storage solution is a 50/50 mixture of 3 molar KCl and pH4 calibration solution. Interestingly, that produces a 1.5 molar KCl solution that falls within the 1 to 2 molar storage solution that I have recommended in the past.
He did admit that the type of usage has an impact on a probe's lifespan. Some use can kill a probe in a couple of days, while other usage could allow a probe to last up to 6 years. My experience is that brewery use with proper sample cooling and probe storage will give at least a year's life and homebrewers could approach that 6 year lifespan.
Remember that the probe's bulb is glass and you can easily break it if you abuse it or drop it. Treat the probe like it is the fragile 'light bulb' that it is and you should be able to maximize your equipment investment.
Enjoy!
Why so much calcium in some brewing waters?
As pointed out in articles published in The New Brewer and Zymurgy, high calcium content is not always desirable. For ale brewing, 50 ppm Ca is typically adequate to promote adequate flocculation and clearing. But the calcium content can be much lower when brewing lagers. So why do some profiles in Bru'n Water have much more than 50 ppm Ca???
The Pale Ale profile has very high calcium content due to our desire to also have a lot of sulfate in that water. Sulfate is an important component for helping the beer finish dry and allowing the bittering and hops to stand out. That sulfate anion has to be paired with some cation. You generally have the choice of calcium, magnesium, or sodium cations. Since sodium and sulfate don't taste very good together at high concentration, your choices are functionally limited to Ca and Mg. This is a reason that the magnesium content of the Pale Ale profile is somewhat high. Epsom salt (MgSO4) use in the Pale Ale profile enables the brewer to avoid adding too much calcium while still boosting the sulfate content to the high levels that are often desired in a Pale Ale. You just have to be careful to avoid adding too much Mg with the Epsom salt addition since it can have negative taste effects as its concentration in water exceeds 40 ppm Mg.
Adding magnesium to brewing water is typically not necessary since malt provides all the Mg that the yeast need. But in the case of highly mineralized water profiles such as Pale Ale profile, the use of Epsom salt helps avoid excessive calcium content in the brewing water which can prematurely flocculate some yeasts. It is a worthwhile trade-off if you want plenty of sulfate in your brewing water.
Practical Guide to Calcium Chloride Solutions
The problems with solid calcium chloride were described here in a previous post. A major problem with the solid form is that its strength can vary due to it absorbing moisture from the air. This problem is so bad, that Dow Chemical (a major calcium chloride manufacturer) only quotes ranges of strength for their products. Their ‘anhydrous’ product has only 94 to 97 percent calcium chloride by mass (it should be 100 percent) while their ‘dihydrate’ product has 77 to 80 percent by mass (it should be 75.5 percent). So you can see that knowing how much calcium chloride you are actually adding to your brewing liquor is difficult!
Creating your own calcium chloride solution is a better way to know how much you are actually adding to your liquor. It’s really not that difficult to create your own solution, measure its strength, and use it.
The first thing to be aware of: combining calcium chloride and water produces a LOT of heat. It is a highly exothermic reaction. It is possible to raise the temperature of the combined mixture by 100C or 180F when creating a strong solution. This means you must start with cool or cold water. Do not use hot water or it could boil!
For brewery use, a strong solution may not be ideal since they become more viscous and minor errors in dosing produce greater error in the calcium chloride dose. Something between 10 and 30 percent solution may be more practical. To provide an idea of how much solid calcium chloride to add, about 120 grams of ‘near-anhydrous’ solid per liter of water produces about a 10 percent solution (roughly 1 lb/gal in US units). If the dihydrate form is used, the amount of the solid would need to be about 1/3 greater than for anhydrous. Since we can’t rely on the ‘true’ strength of the solids, we have to mix up a solution and then measure its specific gravity (SG) to figure out the solution’s true strength. It’s best to use relatively pure water for the solution. RO or distilled water is recommended. That avoids adding other unknown ions to the solution.
Once a solution has been mixed up and fully dissolved, it is important to cool the solution to room temperature before measuring its SG. Room temperature is generally considered to be between 20C & 25C (68F & 77F). Since upper measurement limit of typical brewing hydrometers is typically 1.180 or less, we are limited to measuring the SG of 20 percent or weaker strength calcium chloride solutions with a hydrometer. For stronger solutions, we have to use an accurate volume measure like a laboratory graduated cylinder and a precision scale to measure SG. Measure the tare weight of the empty cylinder and then fill it with a precise amount of the solution, say 100 mL. Weigh the filled cylinder and subtract the cylinder’s tare weight to determine the weight of the solution it contains. If the weight is in milligrams (mg), the calculation is easy. For example, the weight of the solution was measured at 120 milligrams, then the SG is: 120mg divided by 100 mL, which is 1.200.
With that SG, we can calculate the percentage strength of the solution with this equation:
% strength (w/w) = – 36.158*(SG)^2 + 183.75*(SG) – 147.47
Yes, that equation may be a little difficult for some users, so Versions 3 and above of Bru’n Water software include the strength calculation above and the ability to employ calcium chloride solutions to deliver a more accurate and reliable calcium chloride dose to your brewing liquor. Don’t be left wondering if your calcium chloride solids have absorbed moisture. Convert to liquid!
Early Brewing Film
Here is a film showing the brewing process at a German brewery in 1930. An interesting view of how things were done almost a century ago and how things haven't changed. Double decoction, coolship, cascade chiller, kegging and bottling lines. Enjoy!
Why Ca and Mg Are Not Substitutes For Acid in Sparging Water
An interesting question came up recently. "Why can't we consider the acidifying effect of calcium and magnesium salts in the sparging water when we calculate its acid dose?"
As you should know, calcium and magnesium ions react with phytin compounds from malt to help acidify the mash and reduce its pH. In addition, we know that sparging water should have low alkalinity to reduce the possibility of tannin and silicate extraction during sparging. So it could make sense that we should include the acidifying effect of calcium and magnesium when figuring out how much acid we need to neutralize water alkalinity.
However there is an important factor to recognize from the sparging process: that is that the amount of phytins is substantially reduced during the sparging process and that acidifying effect is also reduced. For brewers that continuously (or 'fly') sparge their grist, the amount of phytins in the mash fall to near zero and that acidifying effect disappears at the end of the runoff. That is precisely the time when the mash needs low alkalinity sparging water to avoid tannin and silicate extraction and those calcium and magnesium ions would not produce an acidifying effect. So, we do need to calculate our sparging water acidification without considering the benefit of the water's calcium and magnesium content.
Batch spargers should also follow this approach since a significant quantity of the mash phytins will have been drained away with the initial wort runoff and there may not be enough phytin left to create the acidification effect in the subsequent mashing water addition.
In brewing, acid is your friend. Enjoy!
Dealing with Variable Water Supplies
Plenty of water supplies are drawn from multiple sources and sometimes the mineral content of those supplies differ. Differing water quality can affect the brewing process and beer quality. This is a discussion of how you might monitor and deal with this problem.
The first thing to understand, is if your water supply has the potential to change. A discussion with the water provider can help you understand if the sources change and if the water quality varies. Often, the provider monitors the water quality and can tell you if there is much variation in the water. If it does vary, you need to be ready to test and monitor your incoming water to assess how that will affect that day's brewing.
There are three relatively simple tests that can be performed quickly and inexpensively in the brewery: Total Dissolved Solids (TDS), Calcium and Magnesium Content, and Alkalinity testing. TDS can be quickly measured using a portable electronic device that is inexpensive and fairly reliable. If the water sources vary between high and low TDS sources, a TDS meter will be a very effective monitoring tool. If there is variation, you should then conduct the following tests.
Calcium, magnesium, and alkalinity tests can be performed fairly accurately using colorimetric (color-changing) test kits. They often have the ability to discern those parameters to 10 ppm or less. That should be sufficient for most brewing use. For professional breweries, high-quality test kits can be obtained from suppliers such as Hach and Lamotte. For homebrewers, test kits intended for Aquarium hobbyists by suppliers such as Salifert can provide sufficient accuracy. In either case, the tests only take a few minutes to perform and the results are immediately available.
With that information, the brewer can revise their water report input to reflect the testing results. With the calcium, magnesium, and alkalinity results, the main components affecting mash and wort pH will be known. The only things not precisely known will be the flavor ion content (Na, SO4, and Cl).
For some waters, the main changes are the calcium, magnesium, and alkalinity and the flavor ions don't change much. It is OK to assume they are similar to their typical values in that case. If the water source TDS changes drastically, then it may be best to proportionally change all the ion concentrations based on the TDS reading and its relation to the TDS value from your original water report. The supporter's version of Bru'n Water includes a TDS calculation on the Water Report Input sheet to help the user understand what that value is for their water report.
While dealing with a variable water supply is not easy, it can be accomplished with the relatively simple and inexpensive tools mentioned here!
A Better Way to Store and Use Calcium Chloride
Some of you know that calcium chloride has a high affinity for water and will actually draw it out of the air. That changes the hydration of the mineral and can cause you to add less calcium chloride than you thought you were adding.
To avoid this water problem, it turns out that it is better to join 'em! What I mean is that we can create a calcium chloride solution that we can easily measure the strength of and not have to worry about the strength changing over time.
Calcium chloride is very soluble in water. If you take your solid calcium chloride and dissolve it in distilled water, we produce a solution that will have a higher specific gravity. That specific gravity is directly related to the strength of the solution.
For most brewers, there is a hydrometer somewhere in the brewery for checking wort and beer gravity. That instrument is all you need. One minor limitation is that most brewing hydrometers have a limited upper gravity reading, say 1.118. While you can make much higher gravity calcium chloride solutions than this, you can still work with low gravity solutions. Create a solution with the distilled water and solid calcium chloride and keep the gravity within the range of your hydrometer. Just keep adding solid calcium chloride until you are near the maximum reading capability of your hydrometer. Make sure that all the solids are fully dissolved and measure the solution's gravity and go to Chart 7 in this document.
Then read off what that gravity reading means in terms of the solution strength. That strength value can then be used to calculate the dose of calcium chloride that you need to achieve the calcium and chloride levels in your liquor. While the calculation is not too difficult, it may be more work than most want to contend with. Fortunately, the next supporter's version of Bru'n Water will include the capability to use both solid and liquid forms of calcium chloride.
Enjoy!
pH Meter Adjustments - What is Going On?
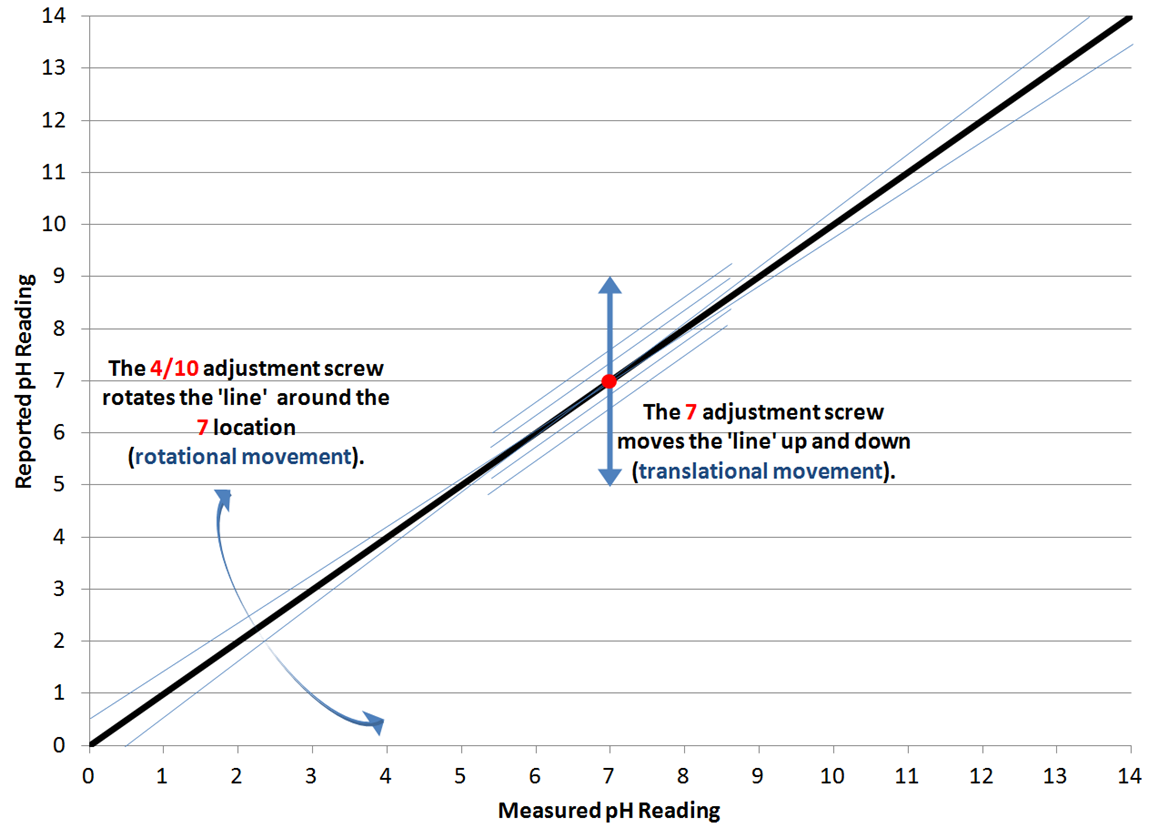
On most moderately-priced pH meters, there are a pair of adjustment screws (typically labeled 7 and 4/10) that are used to calibrate the pH reading that the meter reports. This is a brief explanation of what those screws are doing.
In the figure below is a plot of the measured pH reading versus the reported pH. What those adjustment screws allow the user to do, is alter the reported values to more closely agree with the pH of the measured solution. For meter calibration, we use a pair of solutions with 'known' pH's to adjust the meter's output to match the pH of those known solutions. You either use the 4 and 7 solutions or the 7 and 10 solutions. Since brewing typically observes pH's in the lower range, the 4 and 7 solutions are recommended for calibration in the brewery.
In most cases, you start with the pH 7 solution and use the adjustment screw to move the 'line' in the figure below - up or down so that it reports 7.0. This is a 'translational' movement of the meter response. After the 7 reading has been set, we move to the 4 calibration. When we use the 4/10 adjustment, it is adjusting the slope of the line by 'rotating' the line about the 7 point.
After adjusting the 4/10 screw, it is wise to revisit the 7 calibration solution reading to make sure any adjustment of the 4/10 screw didn't alter the 7 reading. As usual, always rinse your pH probe with distilled water and either blow off or gently dab the probe to remove excess moisture before moving from solution to solution.
Enjoy!
Are Keggles the Best Option for Homebrewing?
Keggles are not an ideal option for homebrewing and moving to a more appropriate kettle configuration is a good idea. The tall, skinny configuration of a keggle presents a number of problems. First, the small bottom area exposed to the heat source reduces the amount of heat transferred to the wort. Secondly, the stainless steel material is also a poor heat conductor. Thirdly, the narrow interior diameter means that the trub is more likely to cover more of the bottom of the keggle and make it more difficult to get all the wort out of the vessel without sucking up trub.
Large aluminum pots can be more suited for use as a kettle. Aluminum has a much better heat transfer coefficient, so more of that heat energy is absorbed from the flame and transferred to the wort. If you get a BIG pot, then the diameter is more likely to enable more of the trub to stay in the middle of the kettle after whirlpooling and that can allow the brewer to get more of their valuable wort out cleanly.
Let's look at kettle configurations in most pro-breweries. They almost always have a MUCH larger diameter in comparison to the depth of wort they contain. For instance, 6- to 12-foot diameters and only 2 or 3 feet of wort depth. Compare that to a keggle with a 1- to 1.5-foot diameter and a 1.5- to 2-foot wort depth.
Homebrewers should consider the pro's guidance when selecting kettles. Opening up kettle material's list to include aluminum can produce real cost savings that can then be converted to purchasing a larger diameter kettle that moves their brewing configuration closer to what the big boys already know. Employing a 15- to 20-gallon, large diameter kettle is a good idea...even for 5 gallon batches.
Enjoy!